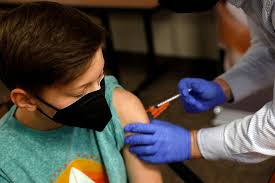
The European Union’s pharmaceuticals regulator approved Pfizer’s coronavirus vaccine for use on children aged 5 to 11 years old on Thursday, paving the way for doses to be given to millions of elementary school students across the continent amid a new wave of infections.
The European Medicines Agency has approved a COVID-19 vaccination for use in young children for the first time.
“An expansion of the indication for the COVID-19 vaccination Comirnaty to cover usage in children aged 5 to 11,” the FDA said.
At least one country dealing with an outbreak of diseases did not wait for the EMA to give its permission. Authorities in Vienna, Austria’s capital, have already begun immunizing children aged 5 to 11. The pandemic is presently centered in Europe, and the World Health Organization has warned that unless immediate action is taken, the continent’s death toll might reach 2 million by the spring.
Before health authorities in member states can begin distributing shots, the EMA green light for the vaccine developed by Pfizer and German business BioNTech must be rubber-stamped by the EU’s executive branch, the European Commission.
Jens Spahn, Germany’s health minister, announced earlier this week that shipping of vaccines for younger children in the EU would begin on December 20.
Pfizer’s kids-sized doses were approved by the US earlier this month, and other nations, including Canada, followed suit.
For elementary school-aged children, Pfizer tried a dose that is a third of the amount given to adults. Dr. Bill Gruber, a Pfizer senior vice president, told reporters in September that youngsters aged 5 to 11 years old acquired coronavirus-fighting antibody levels that were just as high as teenagers and young adults who received the regular-strength doses.
However, the tests on Pfizer’s vaccination in youngsters were not large enough to discover any rare side effects after the second dosage, such as chest and heart inflammation, which has been reported primarily in older male teenagers and young adults.
COVID-19 has caused more deaths in children aged 5 to 11 than some other diseases, such as chickenpox, did before children were routinely vaccinated, according to American officials.
The EMA announced earlier this month that it has begun reviewing the use of Moderna Inc.’s COVID-19 vaccination for children aged 6 to 11 years old, with a decision expected in two months.
Despite the fact that most children only have moderate symptoms from COVID-19, some public health experts believe that immunizing them should be a top priority in order to prevent the virus from spreading further, potentially leading to the creation of a hazardous new strain.
Researchers argue about how much children have influenced the pandemic’s outcome. According to early studies, they didn’t have much of an impact on viral dissemination. However, some experts believe that youngsters played a key role in spreading contagious variations like alpha and delta this year.
Because children and teenagers have weaker COVID-19 disease than adults, WHO says it’s “less important to vaccinate them than older persons, those with chronic health issues, and health workers,” according to a statement released this week.
It has urged wealthy countries to halt immunizing children and to donate their vaccine doses to developing countries that have yet to provide their first vaccination dose to health personnel and vulnerable people.
Nonetheless, the World Health Organization (WHO) recognized that there are advantages to immunizing children and adolescents that go beyond the immediate health benefits.
“Vaccination that reduces COVID transmission in this age group may help reduce transmission from children and adolescents to older adults, as well as the need for school-based mitigation measures,” the WHO said.